INFECTIOUS DISEASE
SPRIM Health developed and validated an FDA-approved COVID-19 symptoms eDiary that was successfully used to detect efficacy signals for the treatment of COVID-19.
Innovations in Clinical Trials and Decentralized Clinical Trials
Infectious Disease is a therapeutic area in which there is a huge opportunity for trials to improve on the quality and confidence of data collected for efficacy and safety outcomes through the use of decentralized clinical trials (DCTs). Whether detecting an AE/SAE is within a clinical trial or during post-marketing surveillance, the speed and accuracy of eliciting an SAE is critical to vaccine studies. It is common that vaccine studies enroll large numbers, often thousands of patients quickly. Such a quick influx of patients and patient data would be particularly problematic for a manual or non-synchronized system for processing all of the structured and unstructured data. Further, timing becomes critical as drugs/vaccines are accelerated to market and across the globe quickly. Thus, having real time/near real time capture of potential solicited and unsolicited AEs/SAEs from patients through the use of an electronic patient reported outcomes (ePRO) can enable standardized and rapid detection and intervention for safety events. The capacity to detect signal earlier can enable pattern recognition and global insights and acceleration to determining potential risk criteria.
Wearables and Biosensors
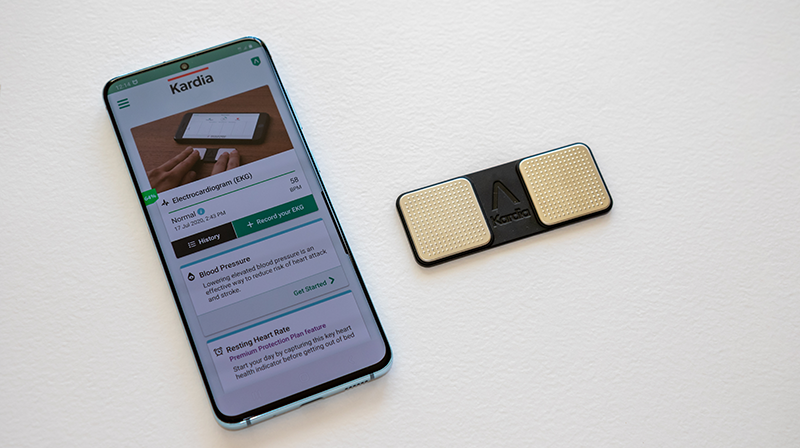
The surge in prevention and treatment studies for COVID-19 has led to the increased use of FDA-approved wearables and biosensors for accurately detecting and monitoring vitals such as heart rate, blood pressure, temperature, body weight, activity and oxygen saturation. At SPRIM Health, we have successfully implemented and integrated a myriad of wearables and biosensors including 6 lead ECGs.
eDiary design/ePRO Instrument Development and Validation
Collection of efficacy outcomes and symptomology can be optimized through the use of ePRO in vaccine and infectious disease interventional clinical trials. Early in the COVID-19 pandemic the FDA quickly generated a guidance document entitled, “Assessing COVID-19-Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment” in Sept 2020 relating to optimal collection of COVID-19 symptoms. The FDA instructed that regarding the “Use of patient-reported outcome (PRO) instruments to assess COVID-19-related symptoms, to
“Use electronic data collection systems with reminders to trial subjects to complete the PRO instrument to minimize missing data and provide time stamps of completion.”
“Conduct an evaluation to ensure the PRO instrument’s basic comprehensibility and usability before implementation in a trial to mitigate risk of poor instrument performance.”

Thus, these recommendations for electronic data capture of patient symptoms and the need to conduct concept elicitation, cognitive debrief and usability testing for COVID-19 symptoms eDiaries was specified. As such SPRIM Health developed and validated an FDA-approved COVID-19 symptoms eDiary that was successfully used to detect efficacy signals for the treatment of COVID-19.
Digital Patient Training
There was always a need for patient training in clinical trials and with the expansion of hybrid and DCTs, it has become even more critical to provide interactive digital multimedia training to patients. Such a training can be provided directly with an app on the patients own smartphone, providing training on relevant topics such as study overview and workflow, roles and responsibilities, expectation setting, accuracy and honesty in reporting, importance of compliance and adherence, video vignettes for how to use specific wearables and/or biosensors and how to report potential AEs and SAEs. SPRIM Health has developed digital patient training for large global DCTs in interventional COVID-19 that improved data quality and led to the achievement of the symptoms-related efficacy primary endpoint.
